Inflammatory Bowel Disease (IBD)
Topics
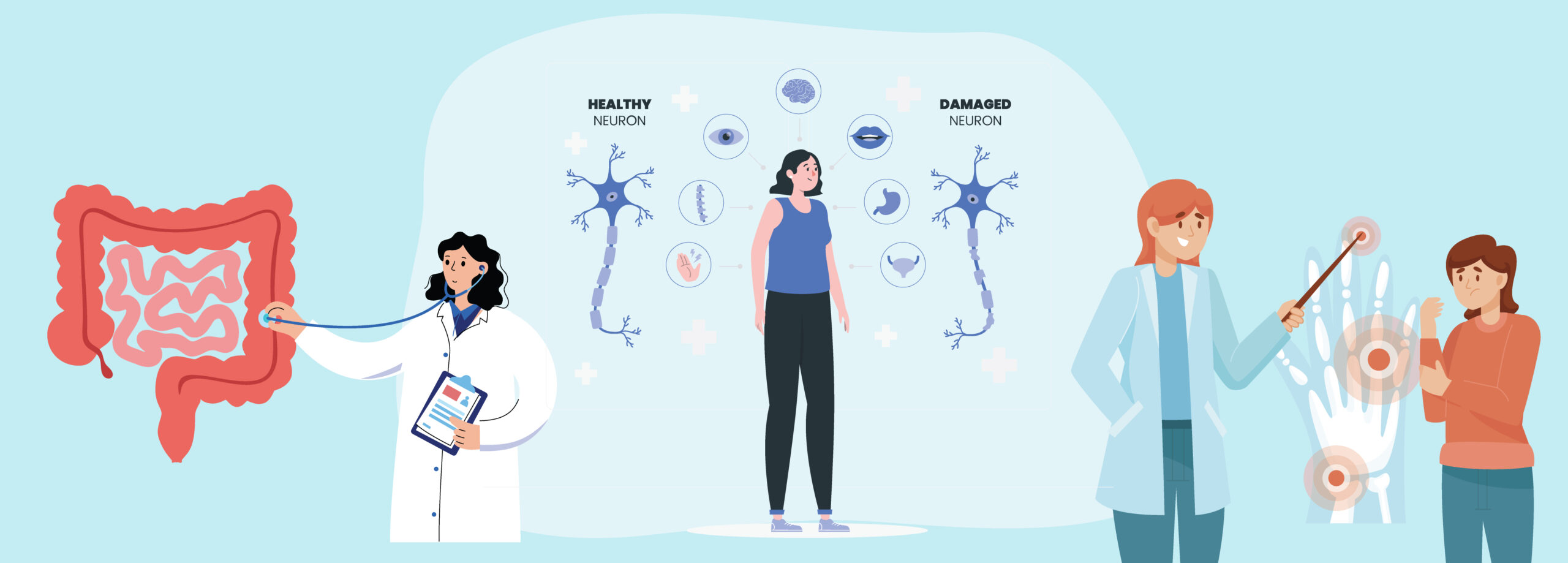
What is IBD?

Medical Leads

Who can participate?

Multiple Sclerosis (MS)
Topics

What is MS?

Medical Leads

Who can participate?
Rheumatoid Diseases (RD)
Topics
What is RD?

Medical Leads

Who can participate?

Patient & Public Involvement
Clinnova will develop a patient & public involvement (PPI) strategy and setup a Patient and Public Advisory Board. This Board will be opened to patients and their family members as well as physicians and members of the general public.
Once implemented, this Patient and Public Advisory Board will help prepare and review information material on Clinnova targeted to patients and the general public, and provide advice on the communication and engagement strategy. Moreover, this Board will provide suggestions for prioritisation of research topics. In the long term, the goal is to co-create research projects together with patients, physicians and researchers.
Welcome to Clinnova
We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. By clicking “Accept”, you consent to the use of ALL the cookies. However you may visit Cookie Settings to provide a controlled consent.
Read More Cookie settings Accept AllREJECT
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |